The Connection Between Diet and Autoimmune Disease

The immune system has two main parts: the innate immune system and the adaptive immune system. The adaptative immune system is more sophisticated; it is capable of identifying and remembering invaders.
Every virus or bacteria has an antigen—a marker on the target tissue that allows the immune system to identify it. The adaptative immune system remembers this antigen after the first time it identifies it, and this allows the system to better defend against the same virus or infection in the future. This is what we mean by developing an immunity.
An autoimmune condition is when the immune system identifies an antigen in its own body’s cells, causing the immune system to start attacking the tissues. Under normal conditions, the immune system is designed to ignore the “self,” but this breaks down when an autoimmune disease develops.
Autoimmune Disease: Why the Immune System Attacks the Body
The mechanism by which the immune system starts to attack its own body is extraordinarily complex. At its heart there is a loss of oral tolerance, which is a fancy term for the immune system’s ability to tolerate self and food.
Oral tolerance is managed by a special type of immune cells called T regulatory cells (Treg). Like other T cells, Treg cells identify antigens, but they identify antigens on our own tissues. They then tell the immune system not to respond to these antigens. This is a highly effective system, but there is one situation where it can naturally break down.
There is another highly inflammatory type of T cell called TH17 cells. It is believed that TH17 were designed to destroy bacterial invaders from the gut which can often mimic the self. Because of the importance of addressing bacterial invasions rapidly and needing to fight off mimic bacteria, there is a value in Treg cells temporarily turning off oral tolerance to allow TH17 to do their thing.
This shift in the balance between Treg and TH17 cells is necessary to address bacterial invasions, but it is meant to be short term. In fact, one theory is that Treg cells mutate into TH17 cells and then mutate back after the infection is addressed. In either case, the elevation in TH17 cells causes inflammation that can damage our own bodies.
When there is a chronic imbalance between Treg and TH17 cells, there can be long-term inflammation that leads to a dysregulation of the immune system that promotes autoimmune conditions. So, it’s no surprise that inappropriate chronically high TH17 concentrations have been found to precede every autoimmune disease.
Diet as Both a Contributor to Inflammation and an Environmental Trigger
It is important to point out that chronically elevated TH17 cells don’t automatically cause autoimmune conditions. The inflammation may “prime” the system, but it is believed that both a genetic susceptibility and an environmental trigger are also needed. An environmental trigger is simply something from outside of our bodies that triggers the autoimmune response.
This is where diet comes in. Some of the foods we consume can be both the environmental trigger and cause the TH17 imbalance that primes the body for an autoimmune condition.
For example, of the over 80 identified autoimmune conditions, the environmental trigger has only been identified in three. One of those three is celiac disease. The environmental trigger is gliadin, which is part of the gluten molecule in wheat.
But gliadin is not just the environmental trigger. In fact, gluten has proven to be remarkably effective at creating all of the conditions necessary to prime the body for an autoimmune disease. Gliadin can break down the gut barrier, which allows bacteria from the gut into our systems—the emergency situation that TH17 cells were designed to fight. But even without that breakdown of the gut barrier, gliadin and another molecule in gluten called wheat-germ agglutin (WGA) have been shown to be very effective at activating two immune cells called dendritic cells and CD14+ macrophages. These cells in turn promote a TH17 imbalance.
Again, this response is appropriate when there is an actual bacterial invasion and the response is short-lived. But genetically susceptible individuals who regularly eat gluten are constantly elevating their TH17 response and priming their bodies for a potential autoimmune disease.
And while gliadin has only been proven to be the environmental trigger in celiac disease, celiac disease has been linked to other autoimmune conditions including Crohn’s disease and type I diabetes—which indicates that the inflammatory priming caused by gliadin may be common to multiple conditions.
Lectins and Autoimmune Disease: More Than Just Gluten
WGA, that powerful inflammatory molecule in wheat, is a type of anti-nutrient called lectins. Lectins are not exclusive to gluten—in fact, most grains have developed lectins as a defense mechanism to protect the plant. Eating them will open the gut, allowing bacteria into the body and activating the immune system. This mechanism is very powerful, which is why eating unprocessed grains will make you very sick.
But, while the lectins in grains are particularly powerful, other plant foods also contain lectins or lectin-like molecules. They include nightshades, legumes such as peanuts, and egg whites.
It’s also important to point out that while it is not a lectin, dietary salt is known to promote general inflammation and a TH17 imbalance.
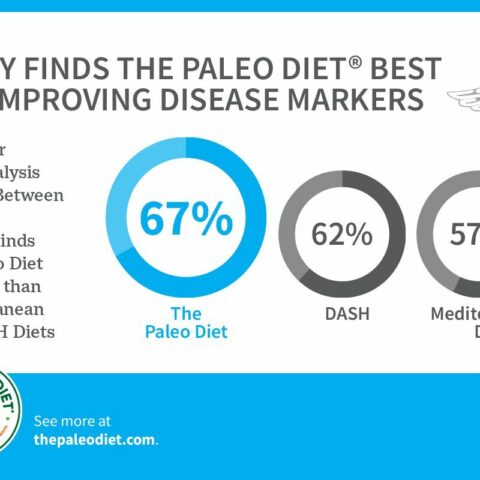
People with an autoimmune condition should try an elimination diet where they completely eliminate grains, the foods listed above, and dairy. Then, over time, reintroduce foods one at a time to see which foods trigger the condition. These changes are in line with The Paleo Diet® and have been successful in helping people with autoimmune conditions like multiple sclerosis.
Trevor Connor, M.S.
Dr. Loren Cordain’s final graduate student, Trevor Connor, M.S., brings more than a decade of nutrition and physiology expertise to spearhead the new Paleo Diet team.
More About The Author