Wheat Series Part 3: How Wheat Mimics Bacteria

It’s a battle that’s been waging for millions of years. Viruses, bacteria, and a variety of pathogens looking for a nice warm home have evolved more and more sophisticated techniques to evade our immune systems. In response, our immune systems developed an array of specialized cells to launch remarkably targeted attacks at these unwanted invaders. In the face of this cellular army, pathogens discovered one of their best weapons is a microscopic form of hide-and-seek.
Viruses mimic our bodies so immune cells pass them by.1, 2 Meningitis hangs out in the nervous system where immune cells dare not go, and HIV takes up home in immune cells themselves—after, of course, dismantling a few defenses. These are all ways of telling the immune system, “Keep moving, nothing to see here.”
But what would happen if instead of looking for a good hiding place, an invader actually tried to signal an immune response? More importantly, why would an invader want to do that?
Well, imagine you’re a plant. When some hungry animal looks at you and says, “lunch,” you can’t really run away. Nor can you fight back. So what do you do? You make sure that after the animal has its meal, it is sick enough to think twice about ever touching one of your brethren.
Enter wheat.
In Part 1 of this series on wheat, I talked about how the normally sluggish digestive immune system can become inappropriately inflamed and lead to disease. Three things can cause this: intestinal permeability (leaky gut), chronic or too high a bacterial load, and dietary antigens. Wheat has the unique distinction of influencing all three.
The first, intestinal permeability, is promoted by wheat through the release of zonulin.3-5 We covered that in Part 2. Let’s get to Part 3: How wheat mimics bacteria.
Of course, you’ve probably already realized that wheat is not bacteria. True. But the same way viruses mimic our bodies, wheat has evolved ways to mimic bacteria. All with the purpose of setting off immune system alarm bells—whether the bacteria are there or not.
How the Immune System Responds to Bacteria
Our bodies actually like bacteria. At least when they stay where they belong—in the gut.6-9 In fact, in Part 1, we talked about how much of our digestive immune system evolved to allow us to live with these bacteria.7, 9-11
It’s when the bacteria—especially the less friendly types such as gram-negative bacteria—get into our bodies that the immune system takes action. As a result, our immune cells have developed critical tools for the sole purpose of hunting down and identifying bacteria inside the body.
Fortunately, the bad gram-negative bacteria have a tell. All over the surface is something called lipopolysaccharide (LPS).12 Antigen presenting cells (APCs) hunt down bacteria using two receptors for LPS called TLR-4 and CD14.12, 13 When LPS binds TLR-4 and CD14, it triggers an immune response.
The diagram below shows the basics of this sophisticated security system,14 but the end result is simple. The immune system spins up and inflammation ensues.
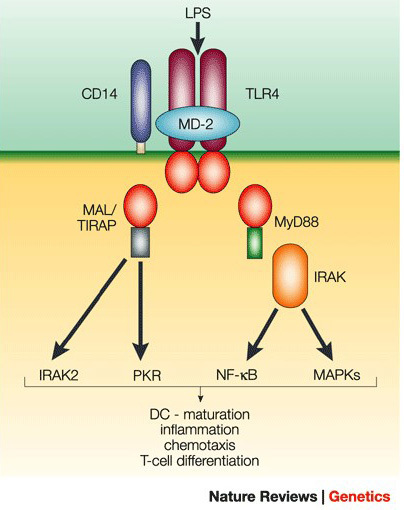
Wheat: The Bacterial Imposter
That subtitle is actually only partially accurate. A better description might be “Wheat: The Boy Who Cried Bacterial Wolf.” The problem is our bodies never learn to ignore the boy.
Wheat has developed a variety of sophisticated techniques for activating the LPS response. But in some cases, it does it differently from LPS, bypassing key regulatory steps such as CD14, which would otherwise prevent inflammation in places we don’t want it.6, 10
A full description of these mechanisms is beyond the scope of this article and probably your boredom limit. So, the following is only a cursory description (but with lots of journal references that will keep the geekiest of you happy).
Mechanism 1: Let Bacteria In
Part 2 gives an in-depth description of how wheat opens up the digestive tract barrier and allows things in our gut to get into our bodies. This includes our intestinal bacteria.15 In other words, wheat actually lets the wolf into the chicken coop and then cries wolf.
Mechanism 2: Molecule Mimicry
Wheat has two molecules that mimic bacteria in our bodies. The first is its own LPS-like molecule, sometimes called LPSw, that has similar effects but admittedly isn’t as potent as the real thing.16, 17 In one study on mice, LPSw was able to promote a bacterial immune response.17
At the barrier of our guts is a special type of immune cell called dendritic cells. Constantly sampling the contents of our digestive tract, they are the on/off switch of the immune system.18 Think of them as Paul Revere riding back to the immune system yelling, “The bacteria are coming!”
Wheat contains molecules that very potently activates dendritic cells called α-Amylase/Trypsin Inhibitors (ATIs).19 They act through TLR-4 on the dendrites. ATIs are responsible for a long-known condition called baker’s asthma, named so because it was common among people who worked with flour.20
Mechanism 4: Promote Inflammation
TLR-4 and CD14 are not strongly expressed in the digestive immune system, making it hard to sound the bacterial alarm in the gut.21-23 In an area of the body that’s exposed to bacteria thousands of times each day, an inflammatory response isn’t something we want.21, 22
So it should be concerning to hear that wheat has developed ways of causing the inflammatory response without bothering with TLR-4 or CD14. The ways wheat does it gets complex. We’ll just touch on them.
First, in several studies, small amounts of gluten were able to flip the dendritic cells’ “on switch” in mice and start an inflammatory response without touching TLR-4.24, 25
Another molecule in wheat (there’s a lot) called wheat germ agglutinin (WGA) can bind and pass right through the gut barrier to interact with immune cells on the other side. WGA then promotes a highly inflammatory response,26, 27 including turning dendritic cells on.
Finally, remember all those antigen-presenting cells in the gut that avoid sounding the bacterial alarm bells by simply not expressing CD14? Gliadin promotes something called IL-15 which is highly effective at activating APCs that don’t express CD14.28-33 And of a variety of foods tested, gliadin was the only one able to so effectively activate these cells.33
What Happens When Your Body Responds to Bacteria That Isn’t There?
That’s a lot of science and frankly we only just skimmed the surface. So here’s the point: Wheat is amazingly effective at activating the bacterial defense mechanisms of our immune cells. More importantly, this response happens in everyone and not just those with celiac disease (though there’s evidence it’s more pronounced in celiacs).29
So what happens when our bodies mount a defense against bacteria that isn’t there? The answer to that question is the focus of the final part to this series. But the short answer is it creates a constant state of inflammation as long as we continue to eat wheat.34, 35
Research is now associating a state of constant inflammation with the onset of nearly all major chronic diseases36 including heart disease,37 Alzheimer’s disease,38 diabetes,39 cancer,40, 41 and overall morbidity.42 But the question remains: Does the inflammation that results from how wheat mimics bacteria also contribute to these conditions?
That’s a question we’ll delve into in the following two installations. But fortunately, by eating a wheat-free Paleo diet, it’s a question you may never have to worry about.
Explore the rest of our Wheat Series:
Part 1: The Digestive Immune System
Part 2: Wheat and Gluten’s Effect on Intestinal Permeability
NEXT: Wheat as a Harmful Dietary Antigen
Part 5: How Wheat Can Trigger Chronic Disease
References
[1]Alcami, A., Viral mimicry of cytokines, chemokines and their receptors. Nat Rev Immunol, 2003. 3(1): p. 36-50.
[2]Amara, A. and J. Mercer, Viral apoptotic mimicry. Nat Rev Microbiol, 2015.
[3]Lammers, K.M., et al., Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology, 2008. 135(1): p. 194-204 e3.
[4]Drago, S., et al., Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol, 2006. 41(4): p. 408-19.
[5]Visser, J., et al., Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci, 2009. 1165: p. 195-205.
[6]Ohnmacht, C., et al., Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cell Microbiol, 2011. 13(5): p. 653-9.
[7]McFall-Ngai, M., Adaptive immunity: care for the community. Nature, 2007. 445(7124): p. 153.
[8]Ivanov, II, et al., Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell, 2009. 139(3): p. 485-98.
[9]Cao, A.T., et al., Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol, 2012. 189(9): p. 4666-73.
[10]Smith, P.D., et al., Intestinal macrophages and response to microbial encroachment. Mucosal Immunol, 2011. 4(1): p. 31-42.
[11]Arrieta, M.-C. and B.B. Finlay, The commensal microbiota drives immune homeostasis. Frontiers in Immunology, 2012. 3.
[12]Kawai, T., et al., Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol, 2001. 167(10): p. 5887-94.
[13]Perera, P.Y., et al., CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol, 2001. 166(1): p. 574-81.
[14]Buer, J. and R. Balling, Mice, microbes and models of infection. Nat Rev Genet, 2003. 4(3): p. 195-205.
[15]Fasano, A., Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev, 2011. 91(1): p. 151-75.
[16]Yamazaki, K., J.A. Murray, and H. Kita, Innate immunomodulatory effects of cereal grains through induction of IL-10. Journal of Allergy and Clinical Immunology, 2008. 121(1): p. 172-178.
[17]Nishizawa, T., et al., Homeostasis as regulated by activated macrophage. I. Lipopolysaccharide (LPS) from wheat flour: isolation, purification and some biological activities. Chem Pharm Bull (Tokyo), 1992. 40(2): p. 479-83.
[18]Williamson, E., G.M. Westrich, and J.L. Viney, Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol, 1999. 163(7): p. 3668-75.
[19]Junker, Y., et al., Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med, 2012. 209(13): p. 2395-408.
[20]Sapone, A., et al., Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med, 2012. 10: p. 13.
[21]Kamada, N., et al., Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest, 2008. 118(6): p. 2269-80.
[22]Nagler-Anderson, C., Tolerance and immunity in the intestinal immune system. Critical Reviews in Immunology, 2000. 20(2): p. 103-120.
[23]Smythies, L.E., et al., Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest, 2005. 115(1): p. 66-75.
[24]Palova-Jelinkova, L., et al., Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J Immunol, 2005. 175(10): p. 7038-45.
[25]Nikulina, M., et al., Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol, 2004. 173(3): p. 1925-33.
[26]Dalla Pellegrina, C., et al., Effects of wheat germ agglutinin on human gastrointestinal epithelium: insights from an experimental model of immune/epithelial cell interaction. Toxicol Appl Pharmacol, 2009. 237(2): p. 146-53.
[27]Gabor, F., M. Stangl, and M. Wirth, Lectin-mediated bioadhesion: binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. J Control Release, 1998. 55(2-3): p. 131-42.
[28]Harris, K.M., A. Fasano, and D.L. Mann, Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clin Immunol, 2010. 135(3): p. 430-9.
[29]Bernardo, D., et al., Is gliadin really safe for non-coeliac individuals? Production of interleukin 15 in biopsy culture from non-coeliac individuals challenged with gliadin peptides. Gut, 2007. 56(6): p. 889-890.
[30]Jelinkova, L., et al., Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett, 2004. 571(1-3): p. 81-5.
[31]Palova-Jelinkova, L., et al., Pepsin digest of wheat gliadin fraction increases production of IL-1beta via TLR4/MyD88/TRIF/MAPK/NF-kappaB signaling pathway and an NLRP3 inflammasome activation. PLoS One, 2013. 8(4): p. e62426.
[32]Thomas, K.E., et al., Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol, 2006. 176(4): p. 2512-21.
[33]Tuckova, L., et al., Activation of macrophages by gliadin fragments: isolation and characterization of active peptide. J Leukoc Biol, 2002. 71(4): p. 625-31.
[34]Nilsen, E.M., et al., Gluten activation of peripheral blood T cells induces a Th0-like cytokine pattern in both coeliac patients and controls. Clin Exp Immunol, 1996. 103(2): p. 295-303.
[35]Antvorskov, J.C., et al., Dietary gluten alters the balance of pro-inflammatory and anti-inflammatory cytokines in T cells of BALB/c mice. Immunology, 2013. 138(1): p. 23-33.
[36]Hotamisligil, G.S., Inflammation and metabolic disorders. Nature, 2006. 444(7121): p. 860-867.
[37]Libby, P., P.M. Ridker, and A. Maseri, Inflammation and atherosclerosis. Circulation, 2002. 105(9): p. 1135-1143.
[38]Akiyama, H., et al., Inflammation and Alzheimer’s disease. Neurobiology of Aging, 2000. 21(3): p. 383-421.
[39]Xu, H.Y., et al., Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation, 2003. 112(12): p. 1821-1830.
[40]Grivennikov, S.I., F.R. Greten, and M. Karin, Immunity, Inflammation, and Cancer. Cell, 2010. 140(6): p. 883-899.
[41]Coussens, L.M. and Z. Werb, Inflammation and cancer. Nature, 2002. 420(6917): p. 860-867.
[42]Krabbe, K.S., M. Pedersen, and H. Bruunsgaard, Inflammatory mediators in the elderly. Exp Gerontol, 2004. 39(5): p. 687-99.




