Wheat Series Part 1: The Digestive Immune System

With a rapidly growing body of nutrition science covering everything from dietary proteins to microflora composition to caloric expenditure and cell bioenergetics, it’s surprising that still one of the hardest arguments to counter remains, “I’ve always eaten it and I’m fine.” It’s a point my 97-year-old grandmother likes to make every time she asks me about my research.
Let me tell you, arguing with a 97-year-old about health is not easy.
The epidemiological version of the “I’m fine” argument is an assertion we hear a lot: while evidence exists that people with celiac disease cannot eat wheat, there is no proof that consuming a gluten-free diet will benefit the rest of the population.1, 2
Celiac sufferers can’t eat wheat. We know that. But it certainly appears that most people can have their bagel, get on with their days, and be just fine—even live to see a century. So it certainly appears that the “I’m fine” argument holds up on the surface. The underlying danger, however, is that the term “fine” is so remarkably subjective.
Take the case of tennis player Novak Djokovic. He went gluten-free in 2011 and then proceeded to have the most successful season in tennis history reaching number one in the process. He was certainly fine when he was eating wheat. He was just better without it.
So let’s take the subjectivity out of fine. Since we define The Paleo Diet® as eating what we were designed to eat, perhaps a Paleo way of defining “fine” is functioning the way we were designed to function. When looked at this way, there is in fact a great deal of research showing the various ways in which wheat causes our bodies to function abnormally. A select unfortunate few, such as those with celiac disease and diabetes, may take the brunt of it, but none of us function normally eating wheat. None of us are fine.
This article is the first part in our wheat series summarizing current research on wheat and the immune system. The next few pieces will detail how wheat causes our bodies to stop functioning the way they were designed to function and can, ultimately, lead to disease. But to understand the damage, let’s start by examining what our digestive immune system looks like when it’s functioning just fine.
Digestive Immunity
Our digestive immune system is one of the most complex and robust systems in our bodies. Some 50×109 immune cells reside in the gut-associated lymphoid tissue (GALT) which makes up the bulk of our immune cells.3
But why are there so many immune cells in the gut? Because, as the image below shows, the gut is an area of constant stress. The digestive tract is continually bombarded by bacteria, food particles, and pathogens.4,5
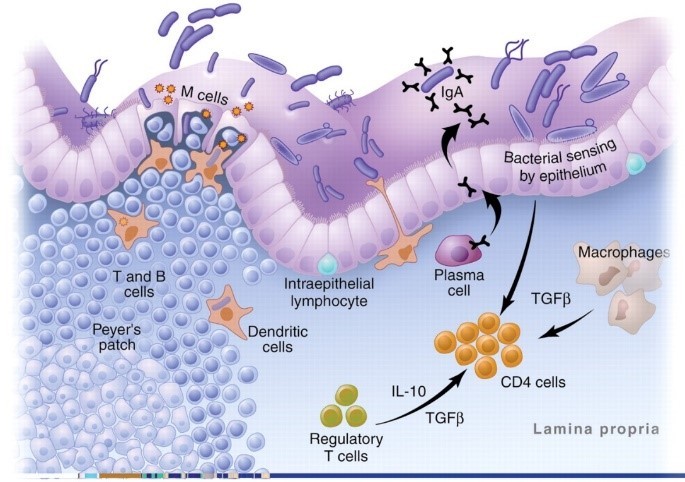
This image is actually a highly simplified version of what goes on in the GALT. The reality is a complex mix of T Cells, monocytes, cytokines, chemokines, interleukins, adhesion molecules, and intricate processes that would have you running for a book on brain surgery to give yourself some light reading.
Don’t worry, we’re not going to cover all that.
We’re just going to focus on a few key concepts that will hopefully prove to be fascinating. But to do that we need to introduce just a few of the important players in the gut.
First is a row of tightly packed cells that keep the contents of the digestive tract from getting into the body. It is our first line of defense and normally very effective at keeping things out.6,7 “Leaky gut” is just a term we use for when this barrier breaks down.
Next in our line of defense are antigen presenting cells (APCs.) They are the macrophages, dendritic, and plasma cells in the image above. These cells “sample” all the food particles, bacteria, and pathogens in the gut and present them to the immune system.
The final players you need to know for this article are T Cells. They are the generals of the immune system. Antigens are presented to the T Cells and then they decide how to respond.
How the Digestive Immune System Responds to Bacteria
Generally when we think about what our immune system deals with, we think about viruses and pathogens and all those nasty things on airplanes and in our kid’s kindergarten classes.
But the truth is, dealing with a pathogen is a rare thing for our digestive immune system. Most of its energy is spent managing our microflora—those beneficial bacteria we pop probiotics and eat yogurt to encourage. We need them for our health. We just also need them to stay in our gut because they aren’t so beneficial inside our bodies.8,9,10,11
If you’re wondering how big a role these bacteria play, remember there are more cells in our microflora than cells in our own bodies. They are so important in fact that several researchers proposed that our digestive immune system evolved not because of pathogens but to allow us to live in harmony with our microflora.5,9,11,12 This is a critical distinction!
If a pathogen or even the normally healthy bacteria in our gut gets into our blood, our bodies mount an immediate and strong inflammatory response.13,14 This inflammation is what causes the aches, fevers, and chills we associate with being sick. The response to a bacterial infection in circulation, though damaging, is necessary and keeps us alive. Fortunately, bacteria rarely gets into our blood.
In the gut, on the other hand, the immune system is exposed to bacteria thousands of times each day. An inflammatory response every time would be deadly.5, 15 There’s even a name for this out of control inflammation: sepsis.16
As a result, the digestive immune system takes a very different tact with our beneficial bacteria. It becomes anergic—meaning it actually blocks inflammation.17,18 Special immune cells in the gut called T regulatory (Treg) cells and a unique type of APC cell actively shut down the inflammatory response and then quietly take out the invading bacteria one by one.3,15
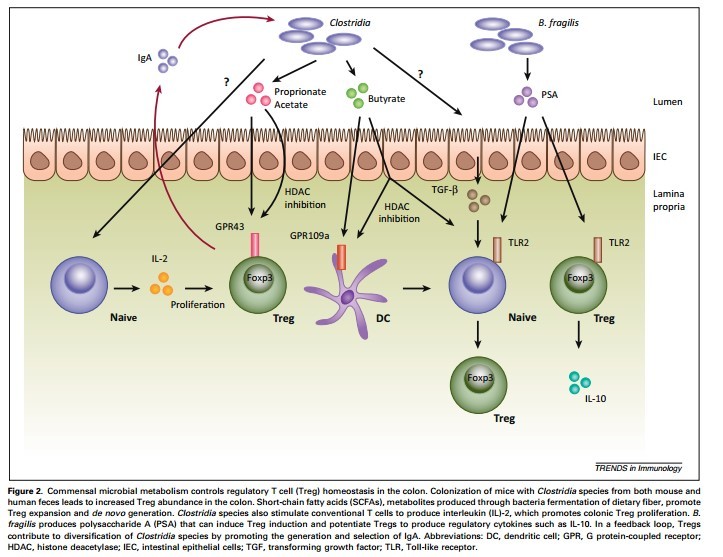
When Dysbiosis Occurs
Problems arise when the bacterial infestation becomes overwhelming or when the inflammation simply doesn’t go away.5,8 As the inflammation continues, the imbalance between anti-inflammatory Treg cells and inflammatory Th17 cells builds on itself until finally the Treg cells can’t control the Th17 cells anymore.24,25,26,27 This imbalance is known as dysbiosis.
No longer protective, Th17 cells can then enter other parts of the body and contribute to a variety of chronic diseases.28,29 such as asthma,30 heart disease,31, 32 and most autoimmune conditions28,33 including celiac disease,34,35 type I diabetes,36,37 Crohn’s disease,38,39 rheumatoid arthritis,29,40 and multiple sclerosis.41
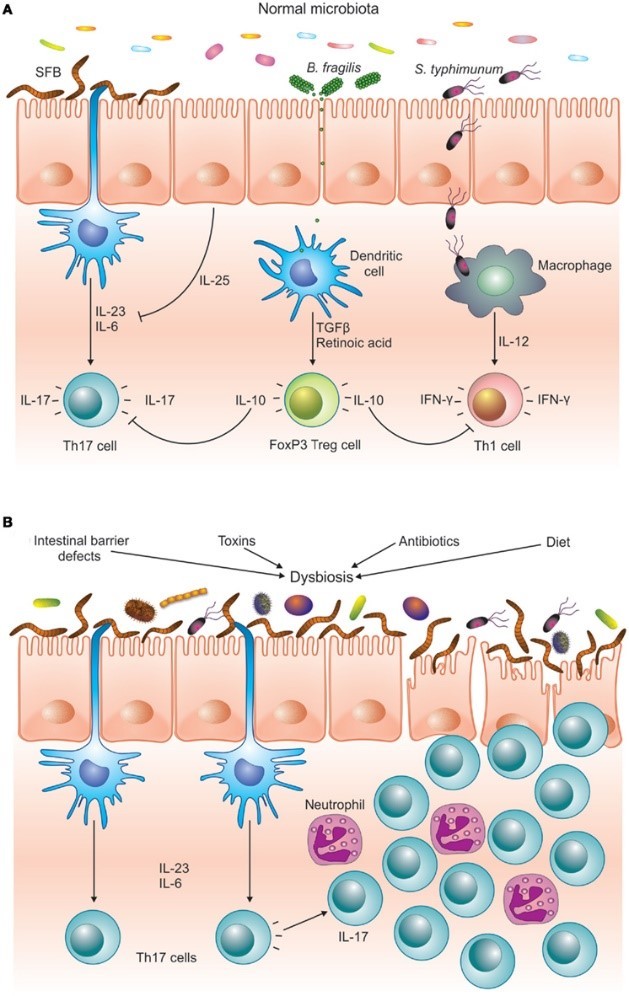
This highly pathogenic Th17 imbalance is a result of an abnormally functioning digestive immune system. Three things are known to cause it:
- Increased intestinal permeability (leaky gut)
- Chronic or too high a bacterial load
- Food particles that can hurt immune function
So now that you’ve plowed through all of that only-interesting-to-people-like-me immune function information, here’s the really fascinating point:
Wheat is the only food we’re aware of that causes all three.
In the remaining articles in this series, I will share with you the surprisingly large number of ways in which wheat breaks down the normal intestinal immune system and leads to damaging Th17 development and dysbiosis.42, 43 More importantly, I will show you that it happens in everyone. In other words, a normally healthy gut exposed to wheat isn’t fine in anyone.
References
[1]Ferch, C.C. and W.D. Chey, Irritable Bowel Syndrome and Gluten Sensitivity Without Celiac Disease: Separating the Wheat From the Chaff. Gastroenterology, 2012. 142(3): p. 664-666.
[2]Gaesser, G.A. and S.S. Angadi, Gluten-Free Diet: Imprudent Dietary Advice for the General Population? Journal of the Academy of Nutrition and Dietetics, 2012. 112(9): p. 1330-1333.
[3]du Pre, M.F. and J.N. Samsom, Adaptive T-cell responses regulating oral tolerance to protein antigen. Allergy, 2011. 66(4): p. 478-90.
[4]MacDonald, T.T. and G. Monteleone, Immunity, inflammation, and allergy in the gut. Science, 2005. 307(5717): p. 1920-1925.
[5]Smith, P.D., et al., Intestinal macrophages and response to microbial encroachment. Mucosal Immunol, 2011. 4(1): p. 31-42.
[6]Visser, J., et al., Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci, 2009. 1165: p. 195-205.
[7]Yu, Q.H. and Q. Yang, Diversity of tight junctions (TJs) between gastrointestinal epithelial cells and their function in maintaining the mucosal barrier. Cell Biol Int, 2009. 33(1): p. 78-82.
[8]Ohnmacht, C., et al., Intestinal microbiota, evolution of the immune system and the bad reputation of pro-inflammatory immunity. Cell Microbiol, 2011. 13(5): p. 653-9.
[9]McFall-Ngai, M., Adaptive immunity: care for the community. Nature, 2007. 445(7124): p. 153.
[10]Ivanov, II, et al., Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell, 2009. 139(3): p. 485-98.
[11]Cao, A.T., et al., Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol, 2012. 189(9): p. 4666-73.
[12]Arrieta, M.-C. and B.B. Finlay, The commensal microbiota drives immune homeostasis. Frontiers in Immunology, 2012. 3.
[13]Koj, A., Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta, 1996. 1317(2): p. 84-94.
[14]Ohl, M.E. and S.I. Miller, Salmonella: a model for bacterial pathogenesis. Annu Rev Med, 2001. 52: p. 259-74.
[15]Smythies, L.E., et al., Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest, 2005. 115(1): p. 66-75.
[16]Bone, R.C., et al., DEfinitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. the accp/sccm consensus conference committee. american college of chest physicians/society of critical care medicine. Chest, 1992. 101(6): p. 1644-1655.
[17]Kamada, N., et al., Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest, 2008. 118(6): p. 2269-80.
[18]Nagler-Anderson, C., Tolerance and immunity in the intestinal immune system. Critical Reviews in Immunology, 2000. 20(2): p. 103-120.
[19]Zeng, H. and H. Chi, Metabolic control of regulatory T cell development and function. Trends in Immunology. 36(1): p. 3-12.
[20]Williamson, E., G.M. Westrich, and J.L. Viney, Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol, 1999. 163(7): p. 3668-75.
[21]Burcelin, R., L. Garidou, and C. Pomie, Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol, 2012. 24(1): p. 67-74.
[22]Yamazaki, K., J.A. Murray, and H. Kita, Innate immunomodulatory effects of cereal grains through induction of IL-10. J Allergy Clin Immunol, 2008. 121(1): p. 172-178 e3.
[23]Reynolds, J.M., et al., Cutting edge: regulation of intestinal inflammation and barrier function by IL-17C. J Immunol, 2012. 189(9): p. 4226-30.
[24]Zhou, X., et al., Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol, 2009. 10(9): p. 1000-7.
[25]Scalapino, K.J. and D.I. Daikh, CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev, 2008. 223: p. 143-55.
[26]Ejsing-Duun, M., et al., Dietary gluten reduces the number of intestinal regulatory T cells in mice. Scand J Immunol, 2008. 67(6): p. 553-9.
[27]Lochner, M., et al., In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med, 2008. 205(6): p. 1381-93.
[28]Kamada, N., et al., Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol, 2013. 13(5): p. 321-35.
[29]Tesmer, L.A., et al., Th17 cells in human disease. Immunological Reviews, 2008. 223: p. 87-113.
[30]Cosmi, L., et al., Th17 cells: new players in asthma pathogenesis. Allergy, 2011. 66(8): p. 989-98.
[31]Taleb, S., A. Tedgui, and Z. Mallat, IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler Thromb Vasc Biol, 2015. 35(2): p. 258-64.
[32]van Bruggen, N. and W. Ouyang, Th17 cells at the crossroads of autoimmunity, inflammation, and atherosclerosis. Immunity, 2014. 40(1): p. 10-2.
[33]Singh, R.P., et al., Th17 cells in inflammation and autoimmunity. Autoimmun Rev, 2014. 13(12): p. 1174-81.
[34]Monteleone, I., et al., Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol, 2010. 184(4): p. 2211-8.
[35]Castellanos-Rubio, A., et al., TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity, 2009. 42(1): p. 69-73.
[36]Kumar, P. and G. Subramaniyam, Molecular underpinnings of Th17 immune-regulation and their implications in autoimmune diabetes. Cytokine, 2015. 71(2): p. 366-76.
[37]Shao, S., et al., Th17 cells in type 1 diabetes. Cell Immunol, 2012. 280(1): p. 16-21.
[38]Elson, C.O., et al., Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology, 2007. 132(7): p. 2359-70.
[39]Brand, S., Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut, 2009. 58(8): p. 1152-67.
[40]Hirota, K., et al., Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med, 2007. 204(12): p. 2803-12.
[41]Du, C., et al., MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol, 2009. 10(12): p. 1252-9.
[42]Antvorskov, J.C., et al., Dietary gluten alters the balance of pro-inflammatory and anti-inflammatory cytokines in T cells of BALB/c mice. Immunology, 2013. 138(1): p. 23-33.
[43]Antvorskov, J.C., et al., Impact of dietary gluten on regulatory T cells and Th17 cells in BALB/c mice. PLoS One, 2012. 7(3): p. e33315.
Trevor Connor, M.S.
Dr. Loren Cordain’s final graduate student, Trevor Connor, M.S., brings more than a decade of nutrition and physiology expertise to spearhead the new Paleo Diet team.
More About The Author