Are Beans Healthy? Why The Paleo Diet Bans Legumes

In the decade since I wrote The Paleo Diet, a question that comes up time and again is, “Are beans healthy? Why can’t I eat beans?” I briefly touched upon this topic in my first book, but was never really able to get into the necessary detail of why you should avoid not only beans, but all other legumes including peanuts and soy.
Now let me bring you fully up to date on recent developments about our understanding of how beans, soy, and other legumes may impact our health.
But most importantly, I’ll show you beyond a shadow of a doubt why legumes are inferior foods that should not be part of any contemporary Paleo Diet.
Toxicity of Uncooked Beans
It may come as a surprise to you, but as recently as 19 years ago imports of red kidney beans into South Africa were legally prohibited because of “their potential toxicity to humans” (63). Many people think about kidney beans as nutritious, plant-based, high-protein foods; few would ever consider them to be toxic poisons. But kidney beans are indeed toxic. Unless they are adequately soaked and boiled, kidney beans and almost all legumes produce detrimental effects in our bodies.
Starting in the early 1970s, a number of scientific papers reported that consumption of raw or undercooked red kidney beans caused nausea, vomiting, abdominal pain, severe diarrhea, muscle weakness and even inflammation of the heart. [42, 52, 60] Similar symptoms were documented in horses and cattle. [8] Further, raw kidney beans were lethally toxic to rats when fed at more than 37% of their daily calories. [24, 27, 51]
Like the proverbial canary in a coal mine, these clues should make us proceed cautiously as we consider the nutritional benefits and/or liabilities of beans and legumes. Before I get into why raw or partially cooked beans, legumes and soy are toxic, I want to first point out the obvious: beans and legumes (even when fully cooked) are not very nutritious when compared with meat, fish and other animal foods.
The Nutrient Content of Beans and Legumes
If we examine the USDA’s My Plate, governmental nutritionists have arbitrarily created five food groups: 1) grains, 2) vegetables, 3) fruit, 4) dairy and 5) protein foods. [61] On the surface, these categories seem reasonable, and I would basically agree that most common foods could logically be placed into one of these five categories except for one glaring exception: protein foods.
Upon more careful inspection of this category, we find the USDA has decided that protein foods should include: 1) meat, 2) poultry, 3) fish, 4) eggs, 5) nuts and seeds and 6) dry beans and peas. I have little disagreement that meat, poultry, fish and eggs are good sources of protein. However, digging a little bit deeper, we soon find that the USDA tells us that these six protein food groups are equivalent and can be used interchangeably with one another (61) – meaning that animal protein sources (meats, poultry, fish and eggs) are nutritionally comparable to plant protein sources (nuts, seeds, dry beans and peas). OK?
It gets better still. A quote from the USDA My Plate recommendations:
“Dry beans and peas are the mature forms of legumes such as kidney beans, pinto beans, black-eyed peas, and lentils. These foods are excellent sources of plant protein, and also provide other nutrients such as iron and zinc. They are similar to meats, poultry, and fish in their contribution of these nutrients. Many people consider dry beans and peas as vegetarian alternatives for meat.” (61).
Let’s let the data speak for itself and really see how “dry beans and peas” stack up to meats, poultry, fish and eggs in terms of protein, iron and zinc as alluded to by the USDA.
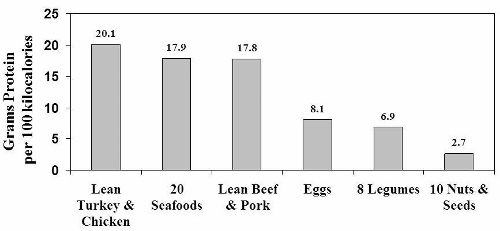
In the figure above [data from (66)] you can see that on a calorie by calorie basis, legumes are utter lightweights when compared to the protein content of lean poultry, beef, pork and seafood. Nuts and seeds fare even worse. Beans, peas and other legumes contain 66% less protein than either lean chicken or turkey, and 61% less protein than lean beef, pork and seafood. What the USDA doesn’t tell us is that our bodies don’t process bean and legume proteins nearly as efficiently as plant proteins – meaning that the proteins found in beans, peas and other legumes have poor digestibility.
The Food and Agricultural Organization (FAO)/World Health Organization (WHO) of the United Nations have devised a protein quality index known as the Protein Digestibility-Corrected Amino Acid Score (PDCAAS). This index reveals that beans and other legumes maintain second-rate PDCAAS ratings which average about 20 to 25% lower than animal protein ratings. [14] So to add insult to injury legumes and beans not only contain about three times less protein than animal foods, but what little protein they do have is poorly digested. Their poor PDCAAS scores stem from a variety of anti-nutrients which impair protein absorption. [20, 29, 44] and from low levels of two essential amino acids (cysteine and methionine). [66]
I don’t know about you, but I have no idea how the USDA concluded that legumes are, “excellent sources of plant protein . . . similar to meats, poultry, and fish in their contribution of these nutrients.”
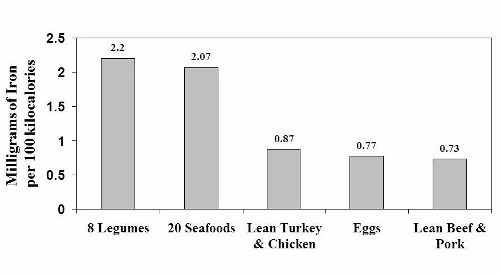
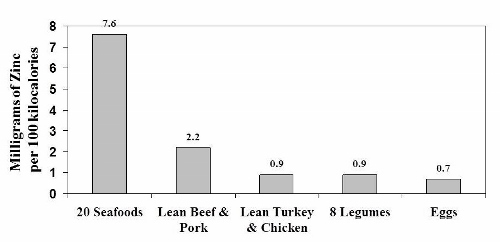
Notice that the iron content of legumes appears to be similar to seafood and about twice as high as in lean meats and eggs. Once again, as was the case with legume protein, this data is misleading because it doesn’t tell us how legume iron is handled in our bodies.
Experimental human studies from Dr. Cook’s laboratory in Switzerland and [30] from Dr. Hallberg’s research group in Sweden [26] have shown that only about 20 to 25% of the iron in legumes is available for absorption because it is bound to phytate. So in reality, the high iron content of legumes (2.2 mg/100 kcal) plummets by 75 – 80%, thereby making legumes a very poor source of iron compared to animal foods. A similar situation occurs with zinc, as phytate and other anti-nutrients in legumes severely reduce its absorption in our bodies. [13, 19, 57]
Given that this information has been known for more than 30 years, it absolutely defies logic how the USDA could misinform the American public by declaring that, “These foods are excellent sources of plant protein, and also provide other nutrients such as iron and zinc. They are similar to meats, poultry, and fish in their contribution of these nutrients.”
Related: Plant Protein vs. Animal Protein: What’s the Difference?
Antinutrients in Beans and Legumes
From the picture I have painted so far, you can see how misleading it can be to evaluate the nutritional and health effects of beans and other legumes by simply analyzing their nutrient content on paper, as the USDA has done. Before we can pass nutritional judgment on any food, it is absolutely essential to determine how it actually acts within our bodies.
Beans are not good sources of either zinc or iron, and they have low protein digestibility because these legumes are chock full of anti-nutrients that impair our body’s ability to absorb and assimilate potential nutrients found in these foods.
As with whole grains, the primary purpose of most antinutrients in legumes is to discourage animals from eating them and to prevent destruction of the plant’s reproductive materials (e.g. its seeds) by microorganisms, insects, birds, rodents and large mammals. [10, 25] We most frequently refer to legume seeds as beans, but don’t forget that peanuts are not really nuts at all, but rather they are legumes. In the table, below I have listed some of the more commonly known legume seeds along with their scientific names.
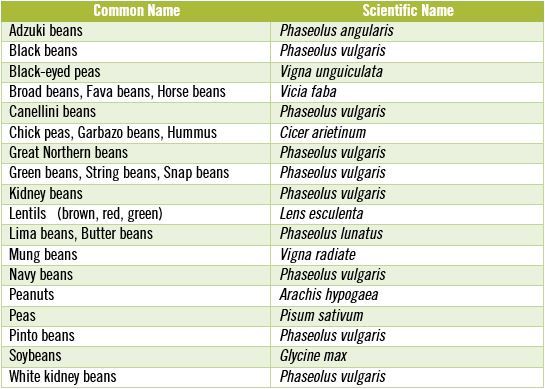
Part of the reason for doing this is to point out that many different versions of the beans we frequently eat actually are the exact same species – and as such contain comparable concentrations of toxic antinutrients. Notice how many times you see the scientific name, Phaseolus vulgaris, repeated in the table above. If you enjoy Mexican food then you have probably tasted Phaseolus vulgaris as either refried beans or black beans, since these two beans are one in the same species, differing only by color. Great Northern beans, green beans, kidney beans, navy beans, pinto beans and white kidney beans also are members of the same species, Phaseolus vulgaris. I bring this information up because all beans that are members of Phaseolus vulgaris contain some of the highest concentrations of antinutrients known.
All Phaseolus vulgaris beans, which are among the most commonly eaten beans, also contain some of the highest concentrations of antinutrients.
Antinutrients in Beans, Legumes, and Soy
The list of antinutrients found in legumes, beans, and soy is seemingly endless and includes: lectins, saponins, phytate, polyphenols (tannins, isoflavones), protease inhibitors, raffinose oligosaccharides, cyanogenetic glycosides, and favism glycosides. I know that this list appears somewhat formidable at first because of all the scientific terms, but don’t be worried – the concepts underlying how these toxins may impair our health are easily understood. Let’s briefly go through this list so you can clearly understand why you should avoid legumes.
Lectins: Block Nutrients and Cause Leaky Gut
All beans and legumes are concentrated sources of lectins. Lectins are potent antinutrients that plants have evolved as toxins to ward off predators. [10] Remember, raw or undercooked kidney beans cause severe cases of food poisoning in humans and were lethally toxic in rats. Although several kidney bean antinutrients probably contributed to these poisonous effects, animal experiments indicate that a specific lectin found in kidney beans was the major culprit. [2, 44] Kidney beans and all other varieties of beans (black beans, kidney beans, pinto beans, string beans, navy beans etc.) within the Phaseolus vulgaris species contain a lectin called phytohemagglutinin (PHA). The more PHA we ingest, the more ill we become. This is why raw beans are so toxic – they contain much higher concentrations of PHA than cooked beans. [4, 23. 46] However, cooking doesn’t completely eliminate PHA, and even small amounts of this lectin are known to produce adverse health effects, providing they can penetrate our gut barrier.
Can Lectins Pass Through the Gut Barrier?
The trick with lectins is that they must bypass our intestinal wall and enter into our bloodstream if they are to wreak havoc within our bodies. So far, no human studies of PHA have ever been conducted. However, in laboratory animals, PHA easily breeches the gut barrier and enters into the bloodstream where it may travel to many organs and tissues and disrupt normal cell function and cause disease. [45, 49] Human and animal tissue experiments reveal that PHA and other food lectins can cause a “leaky gut” and enter circulation. [24, 34, 35, 45, 47, 49, 64, 65] A leaky gut represents one of the first steps implicated in many autoimmune diseases. [67] Impaired intestinal integrity produced by dietary lectins may also cause low level inflammation in our bloodstreams [15, 43, 48, 62] – a necessary step for atherosclerosis (the artery clogging process) and cancer.
Besides kidney beans and other bean varieties within Phaseolus vulgaris species, all other legumes contain lectins with varying degrees of toxicity ranging from mild to lethal. Soybean lectin (SBA) is also known to impair intestinal permeability and cause a leaky gut. [1, 35] Peanut lectin (PNA) is the only legume lectin to have been tested in living humans by Dr. Rhodes’ research group in London. Within less than an hour after ingestion in healthy normal subjects, PNA entered their bloodstreams [64] – whether the peanuts were cooked or not. (Later I will show you how peanuts and PNA are potent initiators of atherosclerosis.)
The lectins found in peas (PSA) and lentils (LCA) seem to be much less toxic than PHA, SBA or PNA, however they are not completely without adverse effects in tissue and animal experiments. [9, 21, 25, 38]
Unfortunately, no long term lectin experiments have ever been conducted in humans. Nevertheless, from animal and tissue studies, we know that these antinutrients damage the intestinal barrier, impair growth, alter normal immune function and cause inflammation.
Saponins: Block Nutrients and Cause Leaky Gut
The term, saponin, is derived from the word soap. Saponins are antinutrients found in almost all legumes and have soap-like properties that punch holes in the membranes lining the exterior of all cells. As was the case with lectins, this effect is dose dependent – meaning that the more saponins you ingest, the greater will be the damage to your body’s cells. Our first line of defense against any antinutrient is our gut barrier. Human tissue and animal studies confirm that legume saponins can easily disrupt the cells lining our intestines and rapidly make their way into our bloodstream. [1, 16, 17, 18, 32] Once in the bloodstream in sufficient quantities, saponins can then cause ruptures in our red blood cells in a process known as hemolysis which can then temporarily impair our blood’s oxygen carrying capacity. [3] In the long term, the major threat to our health from legume saponins stems not from hemolysis (red blood cell damage) but rather from their ability to increase intestinal permeability. [3, 16, 17, 18, 32] A leaky gut likely promotes low level inflammation because it allows toxins and bacteria in our guts to interact with our immune system. This process is known to be is a necessary first step in autoimmune diseases [67] and may promote the inflammation necessary for heart disease and the metabolic syndrome to develop and progress. [68]
The other major problem with legume saponins is that cooking does not destroy them. In fact, even after extended boiling for two hours, 85-100% of the original saponins in most beans and legumes remain intact. [55] On the other hand, by eating fermented soy products such as tofu and tempeh, or sprouted beans you can lower your saponin intake. [39] The table below shows you the saponin content of some common beans, legumes and soy products.
Saponin Concentration in Commonly Consumed Beans & Legumes
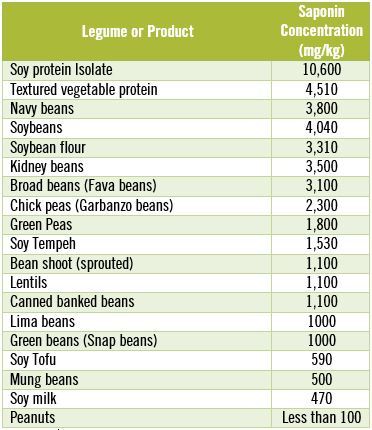
Notice that the concentration of saponins in soy protein isolates is dangerously high.
Saponins, Dietary Supplements, and Athletes
If you are an athlete or anyone else trying to increase your protein intake by supplementing with soy protein isolates, I suggest that you reconsider. A much healthier strategy would be to eat more meats, fish and seafood. These protein-packed foods taste a whole lot better than artificial soy isolates and are much better for your body. If we only eat legumes occasionally, saponin damage to our intestines will quickly repair itself, however when legumes or soy products are consumed in high amounts as staples or daily supplements, the risk for a leaky gut and the diseases associated with it is greatly increased.
Phytate: Blocks Mineral Absorption
We’ve already discussed this antinutrient in great detail, so there is really not much else to say. Because phytate prevents the full absorption of iron, zinc, calcium, magnesium and copper present in legumes and whole grains, then reliance upon these plant foods frequently causes multiple nutritional deficiencies in adults, children and even nursing infants. Boiling and cooking don’t seem to have much effect upon the phytate content of legumes, whereas sprouting and fermentation can moderately reduce phytate concentrations. Also, vitamin C counteracts phytate’s inhibitory effects on mineral absorption. Nevertheless, the best tactic to reduce phytate in your diet is to adopt The Paleo Diet – humanity’s original legume and grain-free diet.
Polyphenols
Polyphenols are antioxidant compounds that protect plants from UV sunlight damage as well as from insects, pests and other microorganisms. Just like sunscreens protect our skin from UV damage, polyphenols are one of the compounds plants have evolved to escape the harmful effects of ultraviolet (UV) radiation from the sun, along with damage caused by animal and microorganism predators. Polyphenols come in many different varieties and forms and are common throughout the plant kingdom. When we eat these compounds, they seem to have both healthful and detrimental effects in our bodies. For instance, resveratrol is a polyphenol found in red wine that may increase lifespan in mice and slow or prevent many diseases. On the other hand, at least two types of polyphenols (tannins and isoflavones) within beans, soy and other legumes may have adverse effects in our bodies (59).
Tannins: Antinutrients
Tannins are bitter tasting polyphenols and give wine its astringent qualities. As with all antinutrients, the more tannin you ingest, the greater is the potential to disrupt your health. Tannins are similar to phytate in that they reduce protein digestibility and bind iron and other minerals, thereby preventing their normal absorption. [29, 59] Some, but not all tannins damage our intestines causing a “leaky gut”. [59]
By now you can see that legumes, beans and soy represent a triple threat to our intestinal integrity since three separate antinutrients (lectins, saponins, and tannins) all work together to encourage a leaky gut.
Isoflavones Mimic Female Hormones
Isoflavones are some of nature’s weirder plant compounds in that they act like female hormones in our bodies. Certain isoflavones which are concentrated in soybeans and soy products are called phytoestrogens – literally meaning, “plant estrogens”. Isoflavones from soy products can cause goiters (an enlargement of the thyroid gland), particularly if your blood levels of iodine are low. Two phytoestrogens in soy called genistein and daidzen produce goiters in experimental animals. You don’t have to develop full blown goiters by these soy isoflavones to impair your health. In a study of elderly subjects, Dr. Ishizuki [31] and colleagues demonstrated that when subjects (average age 61 years) were given 30 grams of soy daily for three months they developed symptoms of low thyroid function (malaise, lethargy, and constipation), and half of these people ended up with goiters.
For women, regular intake of soy or soy isoflavones may disrupt certain hormones that regulate the normal menstrual cycle. In a meta analysis of 47 studies, Dr. Hooper and co-workers [28] demonstrated that soy or soy isoflavones consumption caused two female hormones, follicle stimulating hormone (FSH) and luteinizing hormone (LH), to fall by 20%. The authors concluded, “The clinical implications of these modest hormonal changes remain to be determined.”
I wouldn’t necessary agree with this conclusion, nor would I call a 20% reduction in both FSH and LH “modest”. In one study, seven of nine women who consumed vegetarian diets (containing significant quantities of legumes) for only six weeks stopped ovulating. [69] One of the hormonal changes reported in this study, concurrent with the cessation of normal periods, was a significant decline in luteinizing hormone (LH). Because western vegetarian diets almost always contain lots of soy and hence soy isoflavones, it is entirely possible that soy isoflavones were directly responsible for the declines in LH and the disruption of normal menstrual periods documented in this study.
I have received emails from women all over the world whose menstrual and infertility problems subsided after adopting The Paleo Diet (see Chapter 13). Their stories paint a credible picture that modern day Paleo Diets contain multiple nutritional elements that may improve or eliminate female reproductive and menstrual problems. Unfortunately, scientific validation of these women’s experiences still lies in the future.
Perhaps the most worrisome effects of soy isoflavones may occur in developing fetuses with iodine deficient mothers and in infants receiving soy formula. A recent (2007) paper by Dr. Gustavo Roman [54] at the University of Texas Health Sciences Center has implicated soy isoflavones as risk factors for autism via their ability to impair normal iodine metabolism and thyroid function. Specifically, the soy isoflavone known as genistein may inhibit a key iodine based enzyme required for normal brain development. Pregnant women with borderline iodine status can become iodine deficient by consuming a high soy diet. Their deficiency may then be conveyed to their developing fetus which in turn impairs growth in fetal brain cells known to be involved in autism. Infants born with iodine deficiencies are made worse if they are fed a soy formula. Once again, the evolutionary lesson repeats itself. If a food or nutrient generally was not a part of our ancestral diet, it has a high probability of disrupting our health and that of our children.
Protease Inhibitors: Antinutrients That Block Protein Absorption
Unless you are a biologist by trade or are involved in a very narrow area of human nutrition, very few people on the planet know about protease inhibitors. But I can tell you that when you eat beans, soy or other legumes you should be as aware of protease inhibitors as you are of a radar trap on the freeway – that is – if you don’t want to get a ticket or eat foods that can have unfavorably effects upon your health.
When we eat any protein, we have enzymes in our intestines which break protein into its component amino acids. These enzymes are called proteases and must be operating normally for our bodies to properly assimilate dietary proteins. Almost all legumes are concentrated sources of antinutrients called protease inhibitors which prevent our gut enzymes from degrading protein into amino acids. Protease inhibitors found in beans, soy, peanuts and other legumes are part of the reason why legume proteins have lower bioavailability than meat proteins. [20] In experimental animals ingestion of protease inhibitors in high amounts depresses normal growth and causes pancreatic enlargement. [21, 39, 41] Heating and cooking effectively destroys about 80% of protease inhibitors found in most legumes [5, 11], so the dietary concentrations of these antinutrients found in beans and soy are thought to have little harmful effects in our bodies. Nevertheless, at least one important adverse effect of protease inhibitors may have been overlooked.
When the gut’s normal protein degrading enzymes are inhibited by legume protease inhibitors, the pancreas works harder and compensates by secreting more protein degrading enzymes. Consequently, consumption of protease inhibitors causes levels of protein degrading enzymes to rise within our intestines. One enzyme in particular, called trypsin, increases significantly. The rise in trypsin concentrations inside our gut is not without consequence, because elevated trypsin levels increase intestinal permeability in animal experiments. [53] Once again we see yet another antinutrient found in legumes that contribute to a leaky gut, which as I have explained earlier is not without consequence.
Raffinose Oligosaccharides: Why Beans Cause Flatulence
Here’s another big scientific term for a little problem almost every one of us has had to deal with at one time or another after we ate beans. Beans cause gas or flatulence. Almost all legumes contain complex sugars called oligosaccharides. In particular, two complex sugars (raffinose and stachyose) are the culprits and are the elements in beans that give us gas. [6] We lack the gut enzymes to breakdown these complex sugars into simpler sugars. Consequently, bacteria in our intestines metabolize these oligosaccharides into a variety of gases (hydrogen, carbon dioxide and methane). Beans don’t affect us all equally. Some people experience extreme digestive discomfort with diarrhea, nausea, intestinal rumbling and flatulence, whereas others are almost symptomless. [6] These differences among people seem to be caused by varying types of gut flora (microorganisms).
Cyanogenetic Glycosides: Converts to Cyanide in the Gut
Upon digestion, antinutrients in lima beans called cyanogenetic glycosides are turned into the lethal poison, hydrogen cyanide, in our intestines. Fortunately, cooking eliminates most of the hydrogen cyanide in lima beans. Nevertheless a number of fatal poisonings have been reported in the medical literature from people eating raw or undercooked lima beans. [70]
Although most of us would never consider eating raw lima beans, the problem doesn’t end here. Upon cooking most of the hydrogen cyanide in lima beans is converted into a compound called thiocyanate which you can add to soy isoflavones as dietary antinutrients that impair iodine metabolism and cause goiter. [70] In iodine deficient children, these so-called goitrogens are suspect dietary agents underlying autism. [54]
Favism Glycosides: Toxic Especially for Mediterraneans
Unless you are a bean connoisseur, most of us in the United States have never tasted broad beans which are also known as fava or faba beans. In Mediterranean, Middle Eastern and North African countries broad beans are more popular. Unfortunately, for many people in these countries, particularly young children, consumption of fava beans can be lethal. It has been intuitively known for centuries that fava bean consumption was fatal in certain people. However, the biochemistry of the disease (called favism) has only been worked out in the past 50 years or so. [7]
Favism can only occur in people with a genetic defect called G6PD deficiency. This mutation is the most common human enzyme defect – being present in more than 400 million people worldwide. It is thought to confer protection against malaria. People whose genetic background can be traced to Italy, Greece, the Middle East or North Africa are at a much higher risk for carrying this mutation. If you or your children don’t know if you have the genes causing favism, a simple blood test available at most hospitals and medical clinics can diagnose this problem. Consumption of fava beans in genetically susceptible people causes a massive rupturing of red blood cells called hemolytic anemia and may frequently be fatal in small children unless blood transfusions are made immediately. [7, 71] Not all people with G6PD deficiency experience favism symptoms after they eat broad beans; however if your family background is from the Mediterranean region you may be particularly susceptible.
Although it is not completely known how broad bean consumption causes favism, three antinutrient glycosides (divicine, isouramil and convicine) found in these legumes likely do the damage. [72] These compounds enter our bloodstreams, and in people with the G6PD mutations interact with red blood cells in a manner that causes them to rupture. So, you can now add fava beans along with lima beans to the list of legumes which are lethally toxic.
Peanuts Promote Heart Disease
What’s wrong with peanut oil and peanuts? Most nutritional experts would tell us that they are heart healthy foods because they contain little saturated fat and most of their fat is made up of cholesterol lowering monounsaturated and polyunsaturated fats. Hence, on the surface, you might think that peanut oil would probably be helpful in preventing the artery clogging process (atherosclerosis) that underlies heart disease. Your thoughts were not much different from those of nutritional scientists – that is until they actually tested peanuts and peanut oil in laboratory animals.
Starting in the 1960s and continuing into the 1980s scientists unexpectedly found peanut oil to be highly atherogenic, causing arterial plaques to form in rabbits, rats and primates [73-78] – only a single study [79] showed otherwise. Peanut oil was found to be so atherogenic that it continues to be routinely fed to rabbits to produce atherosclerosis to study the disease itself.
Initially, it was unclear how a seemingly healthful oil could be so toxic in such a wide variety of animals. Dr. David Kritchevsky and co-workers at the Wistar Institute in Philadelphia were able to show with a series of experiments that peanut oil lectin (PNA) was most likely responsible for it artery clogging properties. [36, 37] Lectins are large protein molecules and most scientists had presumed that digestive enzymes in the gut would degrade it into its component amino acids. Consequently, it was assumed that the intact lectin molecule would not be able to get into the bloodstream to do its dirty work. But they were wrong. It turned out that lectins were highly resistant to the gut’s protein shearing enzymes. An experiment conducted by Dr. Wang and colleagues and published in the prestigious medical journal Lancet [64] revealed that PNA got into the bloodstream intact in as little as 1-4 hours after subjects ate a handful of roasted, salted peanuts. Even though the concentrations of PNA in the subject’s blood were quite low, they were still at concentrations known to cause atherosclerosis in experimental animals. Lectins are a lot like super glue – it doesn’t take much. Because these proteins contain carbohydrates, they can bind to a wide variety of cells in the body, including the cells lining the arteries. And indeed, it was found that PNA did its damage to the arteries by binding to a specific sugar receptor. [58] So, the practical point here is to stay away from both peanuts and peanut oil and all legumes.
The Bottom Line
I’d like to make a final departing comment before we leave the topic of beans and legumes. As you adopt The Paleo Diet or any diet, listen to your body. If a food or food type doesn’t agree with you or makes you feel ill or unwell, don’t eat it. I should have listened to my own advice 25 years ago when I was experimenting with vegetarian diets. Whenever I ate beans or legumes, I experienced digestive upset, gas and frequently had diarrhea. Since embracing The Paleo Diet almost 20 years ago, these symptoms have become a thing of the past.
References
1. Alvarez JR, Torres-Pinedo R. Interactions of soybean lectin, soyasaponins, and glycinin with rabbit jejunal mucosa in vitro. Pediatr Res. 1982 Sep;16(9):728-31.
2. Banwell, JG, Howard R, Kabir I, Costerton JW. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Canadian Journal of Microbiology. 1988; 34:1009-13.
3. Baumann E, Stoya G, Völkner A, Richter W, Lemke C, Linss W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000 Feb;102(1):21-35.
4. Boufassa C, Lafont J, Rouanet J M, Besancon P 1986 Thermal inactivation of lectins (PHA)isolated from Phaseolus vulgaris. Food Chem 20 295-304.
5. Buera M P, Pilosof A M R, Bartholomai G B 1984 Kinetics of trypsin inhibitory activity loss in heated flour from bean Phaseolus vulgaris. J Food Sci 49 124-126.
6. Calloway DH, Carol A. Hickey CA, Murphy EL. Reduction of intestinal gas-forming properties of legumes by traditional and experimental processing methods. J Food Sci. 1971; 36: 251-255.
7. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008;371(9606): 64–74.
8. Carmalt J, Rosel K, Burns T, Janzen E. Suspected white kidney bean (Phaseolus vulgaris) toxicity in horses and cattle. Aust Vet J. 2003 Nov;81(11):674-6.
9. Caron, M. & Steve, A.P. Lectins and Pathology, Taylor & Francis, 2000, London.
10. Chrispeels, M.J. & Raikel, N.V. (1991) Lectins, lectin genes, and their role in plant defense. Plant Cell 3, 1-9.
11. Collins J L, Beaty B F 1980 Heat inactivation of trypsin inhibitor in fresh green soybeans and physiological responses of rats fed the beans. J Food Sci 45 542-546.
12. Cordain L, Toohey L, Smith MJ, Hickey MS. Modulation of immune function by dietary lectins in rheumatoid arthritis. Br J Nutr. 2000 Mar;83(3):207-17.
13. Couzy F, Mansourian R, Labate A, Guinchard S, Montagne DH, Dirren H. Effect of dietary phytic acid on zinc absorption in the healthy elderly, as assessed by serum concentration curve tests. Br J Nutr. 1998 Aug;80(2):177-82.
14. FAO/WHO Expert Consultation. Protein Quality Evaluation. Food and Agricultural Organization of the United Nations, FAO Food and Nutrition Paper 51, Rome.
15. Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. Journal of Immunology. 1990;144: 33347-53.
16. Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002 Dec;88(6):587-605.
17. Gee JM, Johnson IT. Interactions between hemolytic saponins, bile salts and small intestinal mucosa in the rat. J Nutr. 1988 Nov;118(11):1391-7.
18. Gee JM, Wal JM, Miller K, Atkinson H, Grigoriadou F, Wijnands MV, Penninks AH, Wortley G, Johnson IT. Effect of saponin on the transmucosal passage of beta-lactoglobulin across the proximal small intestine of normal and beta-lactoglobulin-sensitised rats. Toxicology. 1997 Feb 28;117(2-3):219-28.
19. Gibson RS, Bailey KB, Gibbs M, Ferguson EL. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr Bull. 2010 Jun;31(2 Suppl):S134-46.
20. Gilani GS, Cockell KA, Sepehr E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J AOAC Int. 2005 May-Jun;88(3):967-87.
21. Grant G. Anti-nutritional effects of soyabean: a review. Prog Food Nutr Sci. 1989;13(3-4):317-48.
22. Grant G, More LJ, McKenzie NH, Stewart JC, Pusztai A. A survey of the nutritional and haemagglutination properties of legume seeds generally available in the UK. Br J Nutr. 1983 Sep;50(2):207-14.
23. Grant G, More LJ, McKenzie NH, Pusztai A. The effect of heating on the haemagglutinating activity and nutritional properties of bean (Phaseolus vulgaris) seeds. J Sci Food Agric 1982;33: 1324-1326.
24. Greer F, Pusztai A. (1985). Toxicity of kidney bean (Phaseolus vulgaris) in rats: changes in intestinal permeability. Digestion. 1985 32: 42-46.
25. Gupta YP. Anti-nutritional and toxic factors in food legumes: a review. Plant Foods Hum Nutr 1987;37:201-228.
26. Hallberg L, Hulthén L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr. 2000 May;71(5):1147-60.
27. Hintz HF, Hogue DE, Krook L. Toxicity of red kidney beans (Phaseolus vulgaris) in the rat. J Nutr. 1967 Sep;93(1):77-86
28. Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post- enopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009 Jul-Aug;15(4):423-40.
29. Hughes JS, Acevedo E, Bressani R, Swanson BG. Effects of dietary fiber and tannins on protein utilization in dry beans (Phaseolus vulgaris). Food Res Int 1996;29:331-338.
30. Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr. 1992 Sep;56(3):573-8.
31. Ishizuki Y, Hirooka Y, Murata Y, Togashi K. The effects on the thyroid gland of soybeans administered experimentally in healthy subjects. Nippon Naibunpi Gakkai Zasshi. 1991 May 20;67(5):622-9.
32. Johnson IT, Gee JM, Price K, Curl C, Fenwick GR. Influence of saponins on gut permeability and active nutrient transport in vitro. J Nutr. 1986 Nov;116(11):2270-7.
33. Keukens EA, de Vrije T, van den Boom C, de Waard P, Plasman HH, Thiel F, Chupin V, Jongen WM, de Kruijff B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim Biophys Acta. 1995 Dec 13;1240(2):216-28.
34. Kilpatrick DC, Pusztai A, Grant G, Graham C, Ewen SW. Tomato lectin resists digestion in the mammalian alimentary canal and binds to intestinal villi without deleterious effects. FEBS Lett. 1985;185:299-305
35. Knudsen D, Jutfelt F, Sundh H, Sundell K, Koppe W, Frøkiaer H. Dietary soya saponins increase gut permeability and play a key role in the onset of soyabean-induced enteritis in Atlantic salmon ( Salmo salar L.). Br J Nutr. 2008 Jul;100(1):120-9.
36. Kritchevsky D et al. Influence of native and randomized peanut oil on lipid metabolism and aortic sudanophilia in the vervet monkey. Atherosclerosis 1982;42:53-58.
37. Kritchevsky D, Tepper SA, Klurfeld DM. Lectin may contribute to the atherogenicity of peanut oil. Lipids 1998 Aug;33(8):821-3
38. Liener IE. Nutritional significance of lectins in the diet. In The Lectins: Properties, Functions, and Applications in Biology and Medicine, pp. 527-52 [I.E. Liener, N. Sharon, I.J. Goldstein, editors]. Orlando; Academic Press, 1986.
39. Liener IE (1994) “Implications of antinutritional components in soybean foods.” Crit Rev Food Sci Nutr., vol. 34, pp. 31-67.
40. Lochner N, Pittner F, Wirth M, Gabor F. Wheat germ agglutinin binds to the epidermal growth factor receptor of artificial Caco-2 membranes as detected by silver nanoparticle enhanced fluorescence. Pharm Res. 2003 May;20(5):833-9
41. Losso JN. The biochemical and functional food properties of the bowman-birk inhibitor. Crit Rev Food Sci Nutr. 2008 Jan;48(1):94-118.
42. Noah ND, Bender AE, Reaidi GB, Gilbert RJ. Food poisoning from raw red kidney beans. BrMed J. 1980 Jul 19;281 (6234):236-7.
43. Muraille E, Pajak B, Urbain J, Leo O. Carbohydrate-bearing cell surface receptors involved in innate immunity: interleukin-12 induction by mitogenic and nonmitogenic lectins. Cell Immunol. 1999 Jan 10;191(1):1-9.
44. Pusztai A, Clarke EM, Grant G, King TP. The toxicity of Phaseolus vulgaris lectins. Nitrogen balance and immunochemical studies. J Sci Food Agric. 1981 Oct;32(10):1037-46.
45. Pusztai A, Greer F & Grant G. Specific uptake of dietary lectins into the systemic circulation of rats. Biochemical Society Transcations. 1989;17, 527-528
46. Pusztai A, Grant G. Assessment of lectin inactivation by heat and digestion. In: Methods in Molecular Medicine: Vol. 9: Lectin methods and protocols. J M Rhodes, JM, J D Milton JD (Eds). Humana Press Inc. Totowa, NJ, 1998.
47. Pusztai A, Ewen SW, Grant G, Brown DS, Stewart JC, Peumans WJ, Van Damme EJ, Bardocz S. Antinutritive effects of wheat-germ agglutinin and other N-acetylglucosamine-specific lectins. Br J Nutr. 1993 Jul;70(1):313-21
48. Pusztai A.. Dietary lectins are metabolic signals for the gut and modulate immune and hormone functions. European Journal of Clinical Nutrition. 1993;47: 691-99.
49. Pusztai A, Ewen SWB, Grant G, Peumans WJ, Van Damme EJM, Rubio LA, Bardocz S. Plant (food) lectins as signal molecules: Effects on the morphology and bacterial ecology of the small intestine. In Lectin Reviews, Volume I , pp. 1-15 [D.C. Kilpatrick, E. Van Driessche, T.C. Bog-Hansen, editors]. St. Louis: Sigma, 1991.
50. Pusztai A, Grant G, Spencer RJ, Duguid TJ, Brown DS, Ewen, SWB, Peumans WJ, Van Damme EJM, Bardocz S. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. Journal of Applied Bacteriology. 1993;75: 360-68.
51. Rattray EAS, Palmer R, Pusztai A. Toxicity of kidney beans (Phaseolus vulgaris L.) to conventional and gnotobiotic rats. Journal of the Science of Food and Agriculture. 1974; 25:1035-40.
52. Rodhouse JC, Haugh CA, Roberts D, Gilbert RJ. Red kidney bean poisoning in the UK: an analysis of 50 suspected incidents between 1976 and 1989. Epidemiol Infect. 1990 Dec;105(3):485-91.
53. Róka R, Demaude J, Cenac N, Ferrier L, Salvador-Cartier C, Garcia-Villar R, Fioramonti J, Bueno L. Colonic luminal proteases activate colonocyte proteinase-activated receptor-2 and regulate paracellular permeability in mice. Neurogastroenterol Motil. 2007 Jan;19(1):57-65.
54. Román GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007 Nov 15;262(1-2):15-26
55. Ruiz RG, Price KR, Arthur AE, Rose ME, Rhodes MJ, Fenwick RG. Effect of soaking and cooking on saponin content and composition of chickpeas (Cicer arietinum) and lentils (Lens culinaris). J Agric Food Chem 1996;44:1526-30.
56. Ryder SD, Smith JA, Rhodes JM. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. Journal of the National Cancer Institute. 1992;84:1410-16.
57. Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002 Dec;88 Suppl 3:S281-5.
58. Sanford GL, Harris-Hooker S. Stimulation of vascular proliferation by beta-galactoside specific lectins. FASEB J 1990;4:2912-2918.
59. Singleton VL. Naturally occurring food toxicants: phenolic substances of plant origin. Adv Food Res. 1981;27:149-242.
60. Tuxen MK, Nielsen HV, Birgens H. [Poisoning by kidney beans (Phaseolus vulgaris)]. Ugeskr Laeger. 1991 Dec 16;153(51):3628-9.
61. U.S.D.A. Choose My Plate.
62. van den Bourne BE, Kijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. Journal of Rheumatology. 1997;24: 55-60.
63. Venter FS, Thiel PG. Red kidney beans–to eat or not to eat? S Afr Med J. 1995 Apr;85(4):250-2.
64. Wang Q, Yu LG, Campbell BJ, Milton JD, Rhodes JM. Identification of intact peanut lectin in peripheral venous blood. Lancet. 1998;352:1831-2
65. Wilson AB, King TP, Clarke EMW, Pusztai A. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in the small intestine. II. Microbiological studies. Journal of Comparitive Pathology. 1980; 90:597-602.
66. Nutritionist Pro Dietary Software. //www.nutritionistpro.com/
67. Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012 Feb;42(1):71-8
68. Piya MK, Harte AL, McTernan PG. Metabolic endotoxaemia: is it more than just a gut feeling? Curr Opin Lipidol. 2013 Feb;24(1):78-85.
69. Pirke KM, Schweiger U, Laessle R, Dickhaut B, Schweiger M, Waechtler M. Dieting influences the menstrual cycle: vegetarian versus nonvegetarian diet. Fertil Steril. 1986 Dec;46(6):1083-8
70. Conn EE. Cyanogenic glycosides. In: Encyclopedia of Plant Physiology. New Series. Volume 8. Secondary plant products [Bell, A.E.; Charlwood, B.V. (Editors)]. 1980 pp. 461-492
71. Schuurman M, van Waardenburg D, Da Costa J, Niemarkt H, Leroy P.Severe hemolysis and methemoglobinemia following fava beans ingestion in glucose-6-phosphatase dehydrogenase deficiency: case report and literature review. Eur J Pediatr. 2009 Jul;168(7):779-82
72. Arese P, Bosia A, Naitana A, Gaetani S, D’Aquino M, Gaetani GF. Effect of divicine and isouramil on red cell metabolism in normal and G6PD-deficient (Mediterranean variant) subjects. Possible role in the genesis of favism. Prog Clin Biol Res. 1981;55:725-46
73. Gresham GA et al. The independent production of atherosclerosis and thrombosis in the rat. Br J Exp Pathol 1960;41:395-402.
74. Scott RF et al. Short term feeding of unsaturated vs. satruated fat in the production of atherosclerosis and thrombosis in the rat. Exp Mol Pathol 1964;3:421-443.
75. Wissler RW et al. Aortic lesions and blood lipids in monkeys fed three food fats. Fed Proc 1967;26:371.
76. Kritchevsky D et al. Influence of native and randomized peanut oil on lipid metabolism and aortic sudanophilia in the vervet monkey. Atherosclerosis 1982;42:53-58.
77. Kritchevsky D et al. Lipid metabolism and experimental atherosclerosis in baboons– influence of cholesterol free, semi-synthetic diets. Am J Clin Nutr 1974;27:29-50.
78. Boyle EM et al. Atherosclerosis. Ann Thorac Surg 1997;64:S47-56.
79. Alderson LM et al. Peanut oil reduces diet-induced atherosclerosis in cynomolgus monkeys. Arteriosclerosis 1986;6:465-74.
Loren Cordain, Ph.D.
As a professor at Colorado State University, Dr. Loren Cordain developed The Paleo Diet® through decades of research and collaboration with fellow scientists around the world.
More About The Author




